Abstract
Introduction: Downregulation or loss of B-cell ALL surface antigens (most notably CD19) in patients with acute lymphoblastic leukemia (ALL) targeted by various therapeutic modalities including CART and bi-specific T-cell engagers (BiTEs) has been implicated in acquired resistance to those therapies. Inotuzumab Ozogamicin (InO) is a calicheamicin-conjugated antibody targeting CD22 on ALL blast cells. In the Phase 3 INO-VATE trial, patients with refractory (R/R) ALL who received InO vs standard of care chemotherapy (SC) achieved greater remission, MRD negativity, and improved survival. This study reports CD22 expression levels at baseline, end of treatment (EOT) and relapse in R/R ALL patients who received InO or SC as salvage therapy and subsequently relapsed; the goal of this analysis was to understand if relapse was associated with changes in CD22 expression.
Methods: The study population consisted of R/R ALL patients who were treated with InO or SC as part of two clinical trials: Phase 1/2 Study 1010 (NCT01363297; Phase 1, Expansion Phase, + Phase 2 at a dosage of 1.8 mg/m2) and Phase 3 Study 1022 (NCT01564784). This analysis incorporated CD22 expression levels at baseline, at end of treatment, and at relapse. Central laboratory flow cytometry was used to assess CD22 expression, which was quantified as % CD22-positive leukemic blasts and as Molecules of Equivalent Soluble Fluorochrome (MESF), a quantitative measure of CD22 density on leukemic blasts. Outcomes are reported in InO (Study 1022 or 1010) and SC patients who responded to treatment (patients who achieved complete remission/complete remission with incomplete hematologic recovery [CR/CRi]) and subsequently relapsed. CR was defined by <5% marrow blasts and the absence of peripheral blood leukemic blasts; with recovery of hematopoiesis.
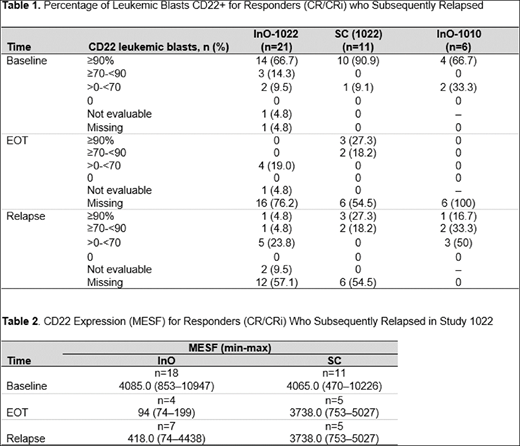
Results: 25 InO patients (Study 1022, n=19; Study 1010, n=6) and 11 SC patients who responded to treatment and subsequently relapsed were evaluable for central-lab CD22 analysis at baseline. Among InO patients who responded to treatment, the majority had leukemic blasts that were ≥90% CD22-positive at baseline (Study 1022, 66.7%; Study 1010, 66.7%); of the evaluable patients at EOT (Study 1022, n=4; Study 1010, n=0), leukemic blast CD22-positivity was >0-<70%. At relapse, all evaluable InO patients had leukemic blasts with detectable CD22-positivity; with blasts that were predominately <90% CD22 positive (Study 1022, n=6/7; Study 1010, n=5/6 [Table 1]). In contrast, the majority of evaluable subjects who received SC and achieved CR/CRi exhibited CD22 positivity that remained ≥90% at EOT and at relapse When CD22 expression was quantified as MESF, a similar trend was evident (Table 2). In Study 1022, InO patients had a median CD22 MESF of 4085.0 at baseline, which declined to 94.0 at EOT and 418.0 at relapse. In contrast, the median CD22 MESF at EOT and at relapse (3738.0 and 3738.0, respectively) was similar to baseline (4065.0) for SC patients.
Conclusions: Among R/R ALL patients who responded to InO treatment and subsequently relapsed, a decrease in CD22 positivity and receptor density was evident from baseline to EOT and relapse, but emergent CD22 negativity was not evident. These results suggest that recurrent disease is associated with decreased CD22 expression, but is not generally attributable to the outgrowth of CD22-negative clones.
Stock:Jazz Pharmaceuticals: Consultancy. Cassaday:Merck: Research Funding; Jazz Pharmaceuticals: Consultancy; Incyte: Research Funding; Seattle Genetics: Other: Spouse Employment, Research Funding; Kite Pharma: Research Funding; Adaptive Biotechnologies: Consultancy; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. DeAngelo:Pfizer Inc: Consultancy, Honoraria; BMS: Consultancy; Glycomimetics: Research Funding; Shire: Honoraria; ARIAD: Consultancy, Research Funding; Blueprint Medicines: Honoraria, Research Funding; Amgen: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria; Takeda: Honoraria; Incyte: Consultancy, Honoraria. Jabbour:Novartis: Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. O'Brien:Pharmacyclics: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Alexion: Consultancy; Sunesis: Consultancy, Research Funding; Regeneron: Research Funding; TG Therapeutics: Consultancy, Research Funding; Celgene: Consultancy; Janssen: Consultancy; Gilead: Consultancy, Research Funding; Aptose Biosciences Inc.: Consultancy; Vaniam Group LLC: Consultancy; Pfizer: Consultancy, Research Funding; Amgen: Consultancy; Acerta: Research Funding; Abbvie: Consultancy; Astellas: Consultancy; Kite Pharma: Research Funding. Stelljes:Novartis: Honoraria; Amgen: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; MSD: Consultancy; JAZZ: Honoraria. Wang:Pfizer: Employment, Equity Ownership. Liau:Pfizer: Employment, Equity Ownership. Nguyen:Navigate BioPharma Services, Inc., a Novartis Subsidiary: Employment. Sleight:Pfizer Inc: Employment, Equity Ownership. Vandendries:Pfizer: Employment, Equity Ownership. Neuhof:Pfizer: Employment, Equity Ownership. Laird:Pfizer: Employment, Equity Ownership. Advani:Novartis: Consultancy; Amgen: Research Funding; Glycomimetics: Consultancy; Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal